Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch
With the Acucy® Influenza A&B Test, clinicians can test and provide fast and accurate results that aid in the treatment of a patient in one office visit, enhancing the patient experience, reducing the spread of infection and improving patient care.

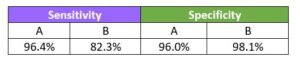
Sensitivity
Specificity
Accurate
Simple
Affordable
CPT Code: 87804QW
*When compared to immunoassay products in market (refer to manufacturer’s instructions for use)
USA - Not available in all countries. Contact us for availability.
Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch