Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch
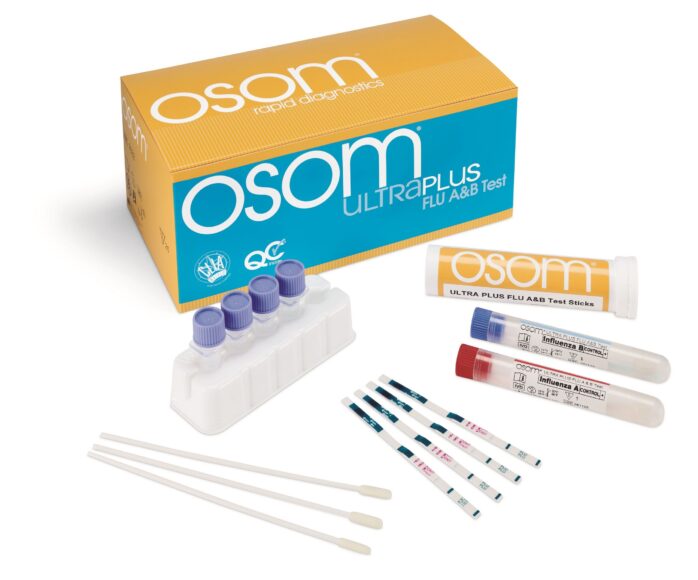
The OSOM Ultra Plus Flu A & B Test is an in vitro rapid qualitative test that detects influenza type A and type B nucleoprotein antigens directly from nasal swab and nasopharyngeal swab specimens obtained from patients with signs and symptoms of respiratory infection.

USA - Not available in all countries. Contact us for availability.
Pricing and ordering details are determined by several factors. Please provide your contact information and we will be in touch shortly with more detailed product information.
Get in Touch